
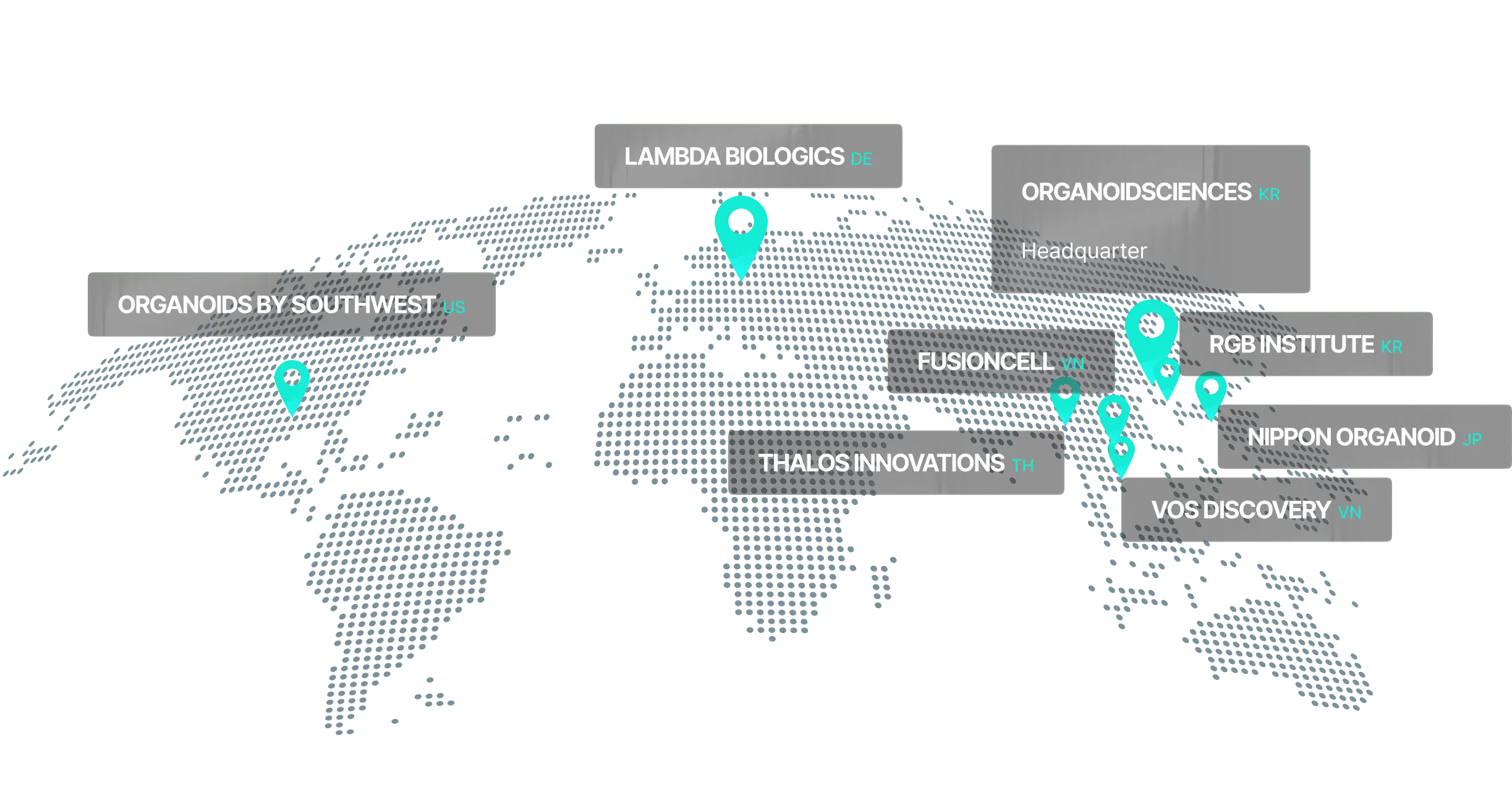
ORGANOIDSCIENCES Overview
Establishing a research hub for technology advancement while expanding production and marketing capabilities worldwide
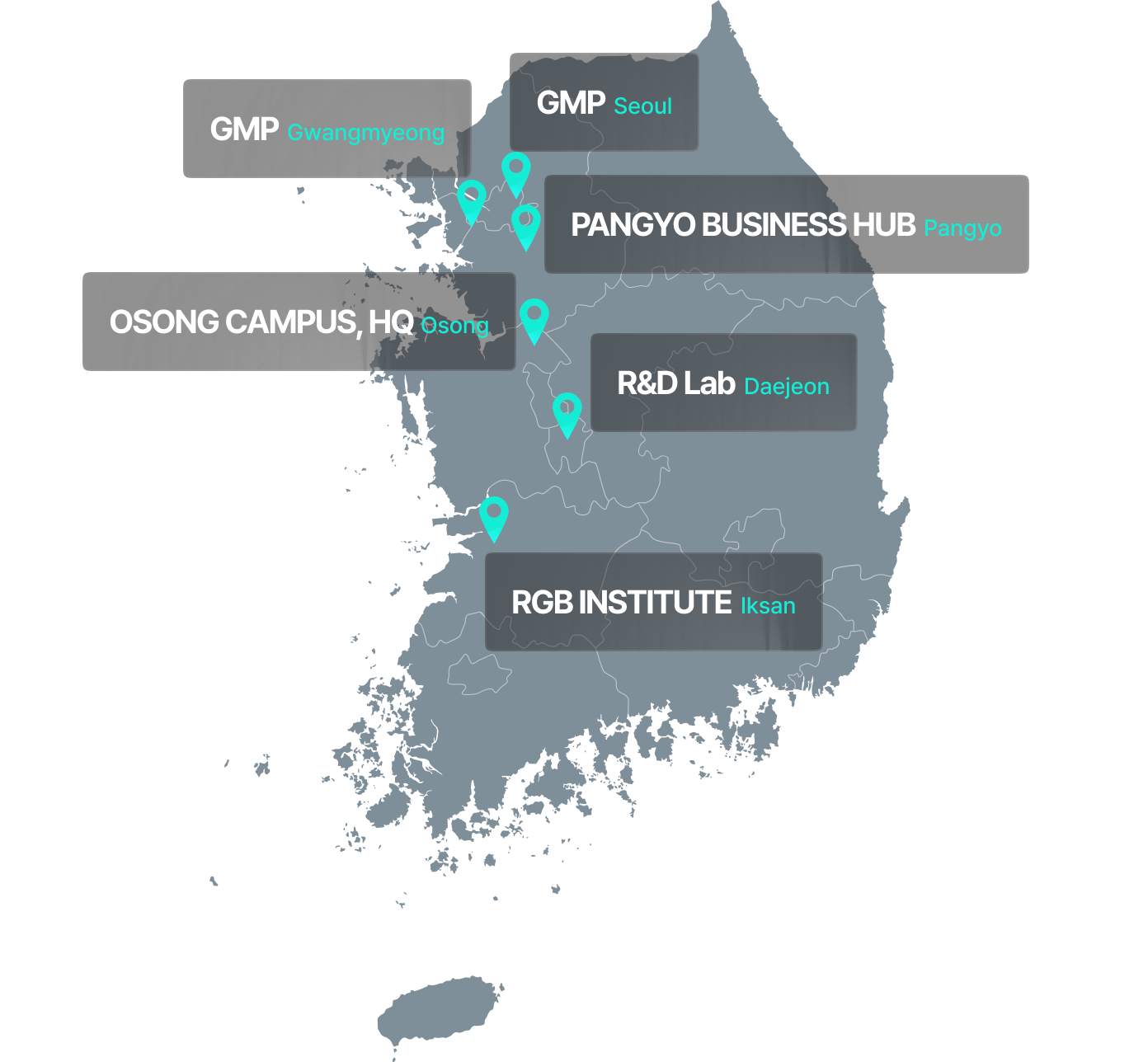
GMP Infrastructure for Organoid-Based Regenerative Therapies
Ensuring stable production and supply through a dedicated GMP facility

Gwangmyeong GMP Center
- Located within SK Technopark in Gwangmyeong
- Rapid facility setup through acquisition of an existing cell therapy GMP facility
- Renovated and optimized for organoid-based production
- Total area: 825 m² (approx. 250 pyeong) with 4 manufacturing suites and a fully equipped quality control room
- Obtained GMP manufacturing license and cell processing facility approval in Q4 2022

ASAN Medical Center GMP Center (Seoul)
- Located within the Biomedical Research Institute at Asan Medical Center
- R&D collaboration agreement signed with Asan Medical Center in June 2021
- Planned area: 560 m² (approx. 170 pyeong) with 2 manufacturing suites and a QC lab (under construction)
- Construction scheduled to begin in April 2025, with completion expected by October 2025
- Backed by Asan Foundation investment through Series A2 and B funding rounds
Core People
Composed of C-Level executives and advisors, each an expert in their respective fields
(R&D, operations, commercialization, management) with 10-30 years of experience with excellence

Sanghoon Oh
CEO (Business & Operations)
- Chairman, Korea Biopharmaceutical Association (2024–Present)
- CEO, CHA Biotech (2019–2025)
- CEO, CHA Health Systems USA (2016–2019)
- CEO, Samsung Fire & Marine Insurance (U.S. Subsidiary) (2014–2016)
- Head of Strategic Planning Team, Samsung Electronics (2005–2014)
- Senior Manager, Office of the Chairman, Samsung Electronics (1999–2005

Jongman Yoo
CEO (Technology & Strategy), Founder
- MD,PhD, CHA University Medical School (2014)
- Associate Professor , CHA University Medical School (2015-)
- Director, CHA Group Organoid Center (2015-)
- Head, Medical Division at CHA Biotech (2018~2022)
- Director & Founder, Organoid Society (2018~2022)
- Head of the 3D Biological Tissue Platform Project Group, Ministry of Trade, Industry and Energy (2020-)

Kyungjin Lee
CTO, Founder (Ph.D.)
Head of R&D, Managing Director
Overall R&D of fundamental technologies
Vice Center Director, Organoid Research Center, Cha Group Comprehensive Research Institute

Dongyeon Hwang
CSO (Ph.D.)
Chief Scientific Officer, Senior Managing Director
Efficacy/evaluation model & analysis method development
Efficacy/evaluation model & analysis method development
Assistant Professor, Harvard Medical School

Seunghye Yang
CMO
Chief Medical Officer
Managing Director R&D of core and applied technologies
Managing Director R&D of core and applied technologies
Foreign Product Development Team Leader, MEDIPOST

Yongseok Lee
Head of Manufacturing Headquarters
Executive Managing Director,
Head of Manufacturing Strategy, CDMO, and Quality Management
- Executive Managing Director, Cell and Gene Therapy Center, CellabMED Inc.
- Director, Recombinant Protein Division, Manufacturing Headquarters, GC Biopharma

Eunjoo Bae
Head of RGB Research Institute
Executive Vice President, Head of RGB Research
Oversees Bio-Convergence Technology and Innovation of Research Infrastructure
Oversees Bio-Convergence Technology and Innovation of Research Infrastructure
- Former Dean, College of Pharmacy, Chonbuk National University
- Adjunct Professor, Chonbuk National University Hospital

Miyoung Son
Ph.D.
Advisory on organoid R&D
Advisory on the enhancement of new material evaluation solutions
Advisory on the enhancement of new material evaluation solutions
Center Director, Stem Cell Convergence Research Center, Korea Research Institute of Bioscience and Biotechnology

Byeongdeok Ye
M.D. / Ph.D.
Advisory on organoid R&D
Advisory on the enhancement of new material evaluation solutions
Advisory on the enhancement of new material evaluation solutions
Director, Inflammatory Bowel Disease Center, Seoul Asan Medical Center

Beomjae Lee
M.D. / Ph.D.
Advisory on ATORM-C development
Clinical trial protocol development and clinical PI
Conducting advanced regenerative medicine clinical research on ATORM-C

Jean–Frederic Colombel
M.D. / Ph.D.
ATORM-C North American clinical design
US clinical trial PI, KOL and infrastructure provision
US clinical trial PI, KOL and infrastructure provision
President, International Organization for Inflammatory Bowel Diseases (IOIBD)

Stefan Schreiber
M.D. / Ph.D.
ATORM-C European clinical design
EU clinical trial PI, KOL and infrastructure provision
EU clinical trial PI, KOL and infrastructure provision
Director of Internal Medicine, Kiel Univ Germany
History
Certifications, Awards & Patents
Year
Certifications, Awards & Patents
- 2018Established ORGANOIDSCIENCES
- 2020Completed Series A investment (KRW 8 billion)
- 2021Secured Series B investment (KRW 41 billion)
- 2022ATORM-C, Approval for Clinical Trial of Advanced Regenerative Medicine
- 2022Established joint venture PODO Therapeutics with Severance Hospital
- 2023ATORM-COrganoid therapy certified as ATMP by EMA
Year
Certifications, Awards & Patents
- 2023First Patient Treatment of ATORM-C in Advanced Regenerative Medicine
- 2023Established VOS DISCOVERY and Partnership Lambda Biologics GmbH
- 2024Certified as “National Advanced Strategic Technology” company
- 2024Selected as a "Pre-Unicorn 2024" by the Ministry of SMEs
- 2025Received the Prime Minister’s Award on National Invention Day
- 2025Approved for KOSDAQ listing under special technology exception
- Commissioned by the Ministry of Food and Drug Safety (MFDS) to develop guidelines for organoid-based regenerative therapies (2023–2025)
- Selected as one of Forbes Korea’s Top 50 Bio Companies in 2024
- Ranked 3rd globally in the number of registered organoid-related patents
- Published organoid-related research with a cumulative impact factor (IF) of 135
- Awarded over KRW 30 billion in national R&D funding
- Commissioned by MFDS for organoid standardization and biobank development (2024–2028)
- First in Korea to register and treat patients with organoid-based regenerative therapy (2023)
Toward a Sustainable Future with ORGANOIDSCIENCES
Join ORGANOIDSCIENCES in leading groundbreaking organoid research and the advancement of next-generation cell therapies.
Be part of the journey of biomedical innovation that is shaping the future of healthcare.
Be part of the journey of biomedical innovation that is shaping the future of healthcare.
Technical Inquiry
bd@organoidrx.com
PR/Marketing inquiry
pr@organoidrx.com
Recruitment inquiry
hr.admin@organoidrx.com
General Inquiry
info@organoidrx.com
