ATORM
Organoid-based regenerative therapeutics platform
Local transplantation
with scaffolds
Patient
Establishment
of organoids
Biopsy by minimal
invasive methods
- Certificate of Advanced Technology Product
- Designation as a National Strategic Technology
Pipeline
Full-scale clinical trials underway for intestinal and salivary gland organoid pipelines
ATORM-C
Intestinal organoid regenerative therapeutic
ATORM-S
Salivary gland organoid regenerative therapeutic
ATORM-E
Endometrium organoid regenerative therapeutic
ATORM-L
Liver organoid regenerative therapeutic
Core Technology
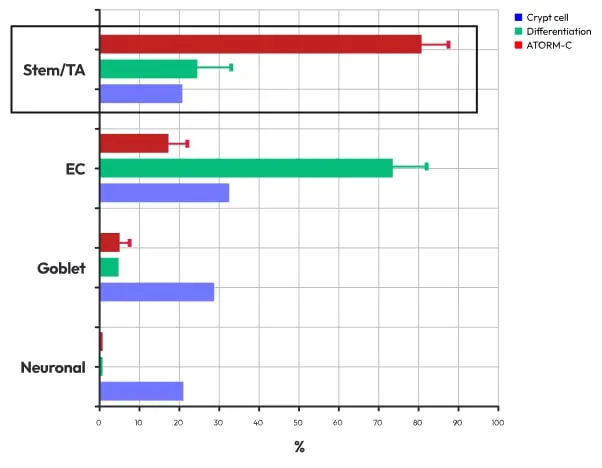
Development of organoid production technology containing high concentrations of stem cells
Our proprietary technology yields intestinal organoids with over four times the stem cell content of native human tissue, significantly enhancing regenerative potential.
Development of delivery method using endoscopy
We developed a user-friendly delivery technique using endoscopy. Therapeutics and fixation materials are injected separately, improving localization while reducing toxicity.
Consistent and optimized organoid culture
ATORM-C Overview
ATORM-C offers fundamental treatment that induces regeneration of damaged intestinal tissue
Refractory intestinal ulcers that do not respond for over a year even after drug treatment
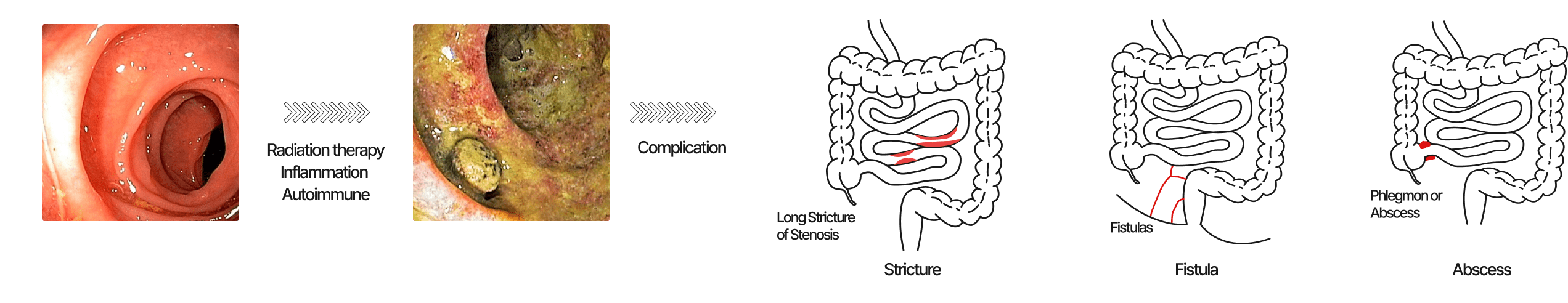
Mechanism and treatment of intractable intestinal ulcers
Inflammation
Treated with various anti-inflammatory agentsMucosal damage
No established treatment currently availableATORM-C Introduction Video
Domestic and global clinical development plans
ATORM-C (Colon Organoids)
Programs (Indications)
(Advanced Regenerative Medicine, Chungbuk Regenerative Bio Special Zone)
Development Stage
Collaboration
- Seoul Asan Medical Center
- Bundang CHA Hospital
- Korea University Medical Center
- Dongguk University Ilsan Hospital
- Yonsei University
Programs (Indications)
Collaboration
- Mount Sinai NY
- UKSH (Universitätsklinikum Schleswig-Holstein)
- Mahidol University
- UKD (University Hospital Dresden ‘Carl Gustav Carus’)
ATORM-S (Salivary Organoids)
Programs (Indications)
Development Stage
Collaboration
- Konkuk University Medical Center
- Samsung Medical Center
Toward a Sustainable Future with ORGANOIDSCIENCES
Be part of the journey of biomedical innovation that is shaping the future of healthcare.
Technical Inquiry
bd@organoidrx.com
PR/Marketing inquiry
pr@organoidrx.com
Recruitment inquiry
hr.admin@organoidrx.com
General Inquiry
info@organoidrx.com
